 Relevancy and Engagement
agclassroom.org/az/
Relevancy and Engagement
agclassroom.org/az/
Lesson Plan
Biofuels and Bioproducts (Grades 9-12)
Grade Level
Purpose
Through a series of activities, students explore fermentation and ethanol production, observe the role of enzymes in fermentation, analyze nutrient values of dent corn, and discover how biofuels are made from plant oils. Grades 9-12
Estimated Time
Materials Needed
Activity 1: Fermentation Factories
- Fermentation Factories student handout, 1 copy per student
- Yeast
- Warm water (95° F/35° C)
- Liquid glucose or crushed glucose tablets
- Corn flour
- Amylase
- Glucoamylase
- Snack-sized bags
- Possible materials for student use:
- Snack-sized bags
- 50 ml water
- 1 tsp. yeast
- ¼ tsp. enzymes (amylase, glucoamylase)
- 1 tsp. sugars (simple & complex) as feedstocks: corn flour, corn starch, corn syrup, honey,
and glucose - Ruler to measure gas volume
- Index card or clipboard to measure gas volume
Activity 2: Ticketase
- Ticketase student handout, 1 copy per student
- 4 sets of 50 single entrance tickets (all connected) per student group
- Timer (optional)
- Blindfold (optional)
Activity 3: Biomass to Sugars
- Biomass to Sugars student handout, 1 copy per student
- Glucose, ground up (3.0 g per station)
- Sweet corn, fresh or frozen, ground up (3.0 g per station)
- Cracked kernel corn or corn flour, ground up (3.0 g per station)
- Test tube holder/hot pads
- 500 ml beakers
- Amylase enzyme solution (1 tsp amylase /100 ml water)
- Glucoamylase enzyme solution (1 tsp glucoamylase / 100 ml water)
- Mortar and pestle
- Hot plates
- Safety goggles
- Hot gloves
- Stirring rods
- Water
- 15 ml centrifuge tubes (10 per group)
- Test tube racks
- Glucose test strips
- Optional: Glucose monitor and test strips
- Scale and weigh boats
- .5 ml disposable pipettes
- Marker/tape
- Scale
- Optional: stopwatch, ice bath, pipette pump with 10 ml serological pipettes
Activity 4: Macromolecules and Fuel
- Macromolecules and Fuel student handout, 1 copy per student
- Hot plate
- Funnel
- Filter paper
- Parafilm or vortex for mixing
- Graduated cylinder (10 mL)
- Benedict’s solution
- Lugol’s iodine solution
- Biuret solution
- Distilled water
- Test tubes and rack
- Cracked corn (ground up)
- Scale or triple beam balance
- Pipette pump
- 10 mL pipettes
- Mortar and pestle
- Test tube holder/hot pads
Activity 5: Corn Mash and Distillation
- Corn Mash and Distillation student handout, 1 copy per student
- Hot plate
- 110V heating mantle
- 100 or 1000 mL distillation apparatus
- Condenser tube
- Dial thermometer
- Graduated cylinders (10, 100 mL)
- Large watch glass covers
- Beakers (100, 250, 600, 1000 mL)
- Deionized water
- Hammered dent corn
- Scale or triple beam balance
- Glass vials with caps (or a small beaker)
- Buffer solution (pH 5)
- Yeast solution (20 g yeast/100 mL water, 49–55° C)
- Amylase solution (3 tsp/100 mL water)
- Glucoamylase solution (3 tsp/100 mL water)
- Funnel
- Thermal gloves
- Glass stir rod
- Cheesecloth/plastic sieve
- Safety glasses
- Hot gloves
- Optional: pipette pump
- Optional: 10 mL serological pipettes
- Optional: aluminum foil
- Optional: paper towels
Activity 6: Biofuels from Plant Oils
- Biofuels from Plant Oils student handout, 1 copy per student
- Methanol
- Sodium hydroxide or Potassium hydroxide
- Glass Jar/lid
- 200 mL beaker
- Magnetic stir bar
- Hot plate/stir option
- Separatory funnel, 250 mL or pint-sized jar with lid
- Ring stand w/ring (not needed if using jars)
- Graduated cylinder
- Serological pump and pipettes
- Distilled water
- Weigh Boats
- Scales
Vocabulary
biofuel: a fuel derived directly from living matter
dent corn: a variety of grain corn high in starch content; named for the dent at the crown of each kernel
distillers grain: a cereal byproduct of the distillation process
enzyme: protein catalyst, which speeds up a specific chemical reaction
ethanol: a fuel produced by fermentation of products high in starch, such as corn
nonrenewable fuels: fuel produced from a source that cannot be readily replaced at the rate it is consumed
renewable fuels: fuels produced from renewable resources such as biofuels and hydrogen fuel
transesterification: chemical process that exchanges the organic R group with the organic R group of an alcohol
Did You Know?
- Corn is the primary grain grown for livestock feed in the United States.3
- More than 90 million acres of land are planted to corn in the United States.3
- Corn is grown for food, livestock feed, ethanol fuel, and other industrial uses.3
Background Agricultural Connections
 Human consumption of fuel is on the rise as both population and affluence steadily increase. Renewable fuels, such as ethanol, can help to decrease the need for non-renewable fuel sources such as crude oil. In addition, ethanol has replaced methyl tertiary-butyl ether (MTBE) as the major octane source in gasoline which has resulted in gasohol blends of up to 10% in almost every pump in the United States. Ethanol is a renewable fuel source that is both energy positive, which means it generates more energy than it consumes, and helps to reduce greenhouse gas emissions. Most gasoline contains 5-15% ethanol as an additive to help to increase octane ratings. Learn more about why ethanol is added to gas.
Human consumption of fuel is on the rise as both population and affluence steadily increase. Renewable fuels, such as ethanol, can help to decrease the need for non-renewable fuel sources such as crude oil. In addition, ethanol has replaced methyl tertiary-butyl ether (MTBE) as the major octane source in gasoline which has resulted in gasohol blends of up to 10% in almost every pump in the United States. Ethanol is a renewable fuel source that is both energy positive, which means it generates more energy than it consumes, and helps to reduce greenhouse gas emissions. Most gasoline contains 5-15% ethanol as an additive to help to increase octane ratings. Learn more about why ethanol is added to gas.
Enzymes work to speed up biological reactions by lowering their activation energy. There are certain conditions that must be met for enzymes to work efficiently. One of these conditions is substrate concentration. Students use tickets to model how various enzymes interact with starch to produce smaller sugar molecules. The tickets represent the substrate. The ends of the ticket strings represent the active sites of the enzymes “Ticketase” and “Glucoticketase.” To catalyze the reaction (tearing groups or single tickets off of the string of tickets), students may only tear a single ticket off at a time if they are Glucoticketase or a group of 2 or 3 tickets off at a time if they are Ticketase. Products and reactants must be dropped back into the pile after every tear off to mix and the process is repeated. A single ticket is the desired product for fermentation and must be torn cleanly off on both sides to count as product.
- Ticketase represents the enzyme Amylase that acts on starch (polysaccharide) to break off a disaccharide (2-sugar molecule) or a trisaccharide (3-sugar molecule).
- Glucoticketase represents the enzyme Glucoamylase that acts on polysaccharides to break off a single sugar molecule.
Commercial production of fuel ethanol in the United States involves breaking down the polysaccharides (starch) present in dent corn into monosaccharides (glucose), feeding these sugars to yeast (fermentation), and then recovering the main product (ethanol) and coproducts (animal feed and carbon dioxide). Ethanol is an alcohol produced through the process of alcoholic fermentation of sugars by yeast. 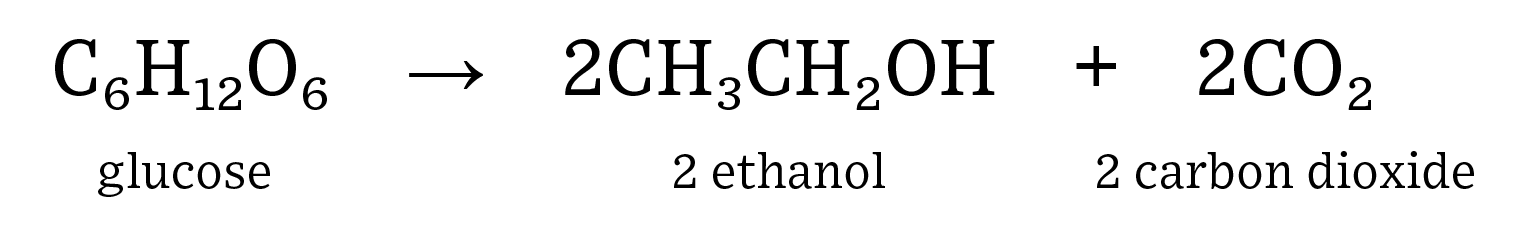 The complex carbohydrates (starch) found in corn must be broken down into monosaccharides for fermentation to be successful. Heating the feedstock can help to break apart carbohydrate bonds but is not 100% successful. Enzymes are used to efficiently cut carbohydrates into simple sugars. For example, amylase breaks down complex carbohydrates into disaccharide molecules: maltose, and glucoamylase breaks down maltose into a monosaccharide molecule: glucose. Glucose is the simple sugar used during fermentation for industrial ethanol production.
The complex carbohydrates (starch) found in corn must be broken down into monosaccharides for fermentation to be successful. Heating the feedstock can help to break apart carbohydrate bonds but is not 100% successful. Enzymes are used to efficiently cut carbohydrates into simple sugars. For example, amylase breaks down complex carbohydrates into disaccharide molecules: maltose, and glucoamylase breaks down maltose into a monosaccharide molecule: glucose. Glucose is the simple sugar used during fermentation for industrial ethanol production.
Commercial production of fuel ethanol in the United States involves breaking down the starch present in corn into simple sugars (glucose), feeding these sugars to yeast (fermentation), and then recovering the main product (ethanol) and coproducts (distillers grains, corn oil, and carbon dioxide). All the remaining nutrients: protein, fat, minerals, and vitamins, are concentrated into distillers dried grain (DDGs), a valuable feed for livestock, and carbon dioxide. In this Activity 3, students will determine the nutrient analysis of dent corn before, during, and after ethanol production.
Commercial production of fuel ethanol in the United States involves breaking down the starch present in corn into simple sugars (glucose), feeding these sugars to yeast (fermentation), and then recovering the main product (ethanol) and coproducts (distillers grains, corn oil, and carbon dioxide). Ethanol is an alcohol produced by yeast from sugars. Fuel ethanol is ethanol that has been highly concentrated and blended with other compounds (gasoline) to render the alcohol undrinkable. For each pound of simple sugars, yeast can produce approximately .5 pounds (0.15 gallons) of ethanol and an equivalent amount of carbon dioxide. The value of corn as a feedstock for ethanol production is due to its large volume of carbohydrates, specifically starch. Starch can be easily processed to break down into simple sugars, which can then be fed to yeast to produce ethanol. Modern ethanol production can produce approximately 2.8-3 gallons of fuel ethanol per bushel of corn. Dry-milled ethanol production uses only the starch portion of the corn, which is about 62% of the kernel. All the remaining nutrients—protein, fat, minerals, and vitamins—are concentrated into dried distillers grains (DDGs), a valuable feed for livestock. Some ethanol plants also remove the corn oil from DDGs to create renewable diesel. Approximately 40% of the United States’ corn crop is used to produce ethanol and distillers grains.
Diesel engines such as trucks, tractors, and heavy motors rely on No. 2 diesel for power. Diesel is commonly made from petroleum distillation. Renewable substitutes for fossil diesel are growing in popularity. These biofuels can be easily made from corn, soy, and other plant oils; animal fats; and waste grease through chemical reactions.
Biofuels refer to liquid or gaseous fuels commonly used for transportation. These are referred to by the United States Department of Agriculture as ‘drop-in fuels’, requiring no modification to engines. Biofuel derived from plant materials is among the most rapidly-growing renewable energy technologies. Ethanol, made mostly from corn starch from kernels using a process called fermentation, is by far the most significant biofuel in the United States. The remaining amount is biodiesel, which is made from vegetable oils (chiefly soy oil) as well as animal fats, waste oils, and greases.1
Biomass-based diesel fuels include biodiesel and renewable diesel.2 Typically, ‘biodiesel’ is the term used for biomass-based diesel from soybean oil, while ‘renewable diesel’ refers to biomass-based diesel from corn oil. Biomass-based diesel burns cleaner than petroleum and is derived entirely from biological sources. Environmental Protection Agency (EPA) research indicates that biomass-based diesel emits 11% less carbon monoxide and 10% less particulate matter than diesel. The Department of Energy and Agriculture found biodiesel reduces net carbon dioxide emissions by 78%. Unlike petroleum diesel, which contains sulfur and carcinogenic benzene, two components regulated by the EPA, biodiesel is nontoxic and biodegradable. They are completely miscible with petroleum diesel, which allows for easy blending. Biomass-based diesel can be combusted in any diesel engine, without needing to modify the engine. Vegetable oils are triglycerides and they have a standard structure. A molecule of any given vegetable oil consists of two parts, a glycerol backbone and three distinct fatty acid chains that stem from the glycerol. Biodiesel is produced using the chemical process known as transesterification. Transesterification occurs when one type of ester, an oil molecule in this case, exchanges an R group with an alcohol. In Activity 6, students will be making biomass-based diesel with oil and methanol. We will also use a catalyst, potassium hydroxide, to speed up the reaction. The combination of catalyst and methanol is called methoxide. The end product is a combination of biomass-based diesel, unreactive methanol, glycerin, and soap. 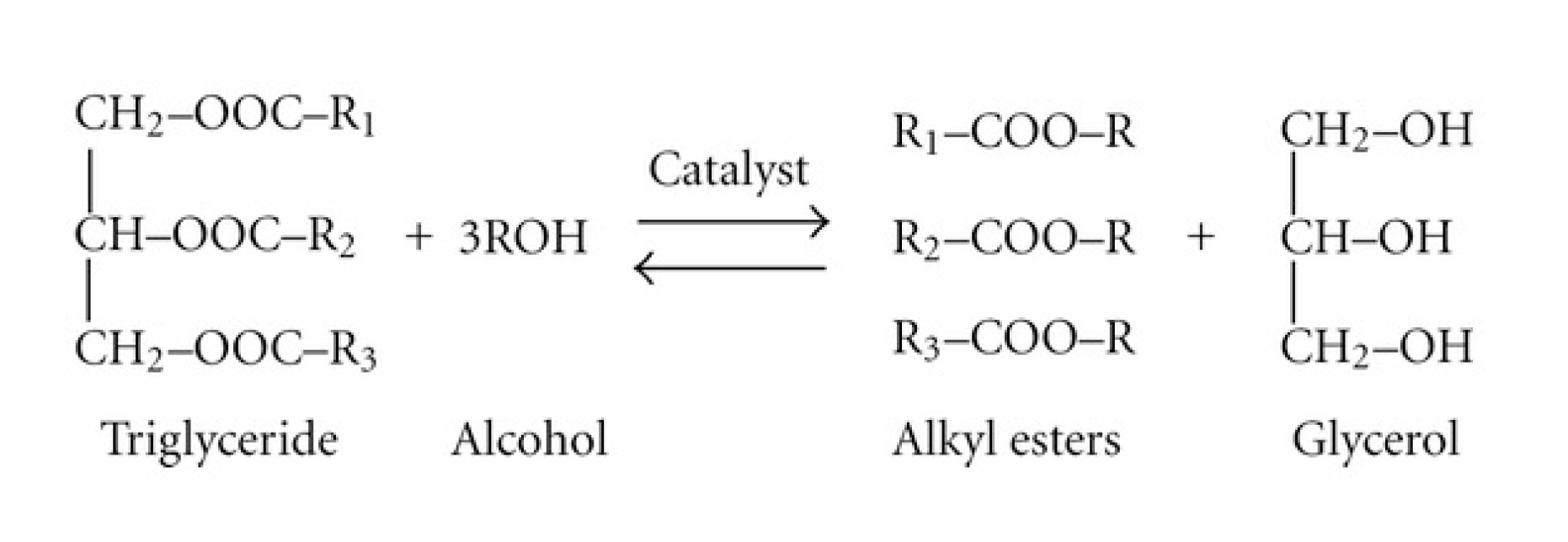
The synthesis is a simple chemical reaction that produces biomass-based diesel and glycerol. The oil is mixed with methanol, while sodium or potassium hydroxide is added as a catalyst. The products separate into two layers with the biodiesel on top. The biodiesel is separated and washed, then it is ready for product evaluation.
In industrial applications, the oil is then refined through a process that we cannot replicate in the lab. Biodiesel undergoes a refinery process similar to petroleum diesel. Renewable diesel results in biodiesel that is stable at low temperatures from a new process that creates a reaction with the feedstock and hydrogen called hydrotreating. Consequently, renewable diesel does not have hydrogen in it, whereas biodiesel does. Renewable diesel also has lower production volumes than biodiesel in the U.S.
Engage
- Show an image of a busy, multilane highway to the students.
- After showing the image, ask students to brainstorm questions individually for 30 seconds to one minute, then share their questions within small groups (3–4 students) for the next two or three minutes.
- Have groups share their questions one-by-one to the large group until all questions are shared.
- Create a Driving Question Board to keep note of the questions, as they will guide the rest of this unit.
- Teaching Tip: For more information on driving question boards, see The Science Teacher (November 2008), Vol 75, No 8.
- Possible questions:
- How many vehicles does the average family in the United States own?
- How many vehicles are in use worldwide?
- Why do we need so many vehicles?
- How many vehicles are in use on average per day?
- How much fuel is consumed on average per day?
- What forms of fuel are available?
- What impact can these fuel types have on the environment?
- Can we extract enough petroleum to meet this growing demand?
- Can we create enough ethanol to create gasohol blends?
- How will human growth impact fuel consumption?
- If no one brings up fuel consumption or fuel production, add your own questions:
- Do we have enough fuel to power these vehicles?
- What types of fuel are available for these vehicles to use?
- What methods are used to produce these fuel types? How will human growth impact vehicle use and fuel consumption in the future?
- To begin investigating these questions, organize the ones that relate to the sheer number of vehicles and the rate at which vehicle use has grown.

Explore and Explain
Activity 1: Fermentation Factories
How can fermentation produce a renewable fuel source?
Preparation: Create the following bags 25–30 minutes prior to class. If possible use warm water (95° F/35° C) to hydrate the fermentation bags. Remove all of the air from the bags, seal, and incubate the bags in a warm location (98.6° F/37° C) for optimum fermentation. Remove the bags from the incubator and ask the students what they are observing. Allow the students to generate discussion with their observations. Do not confirm or deny ideas as you lead the conversation with your students.
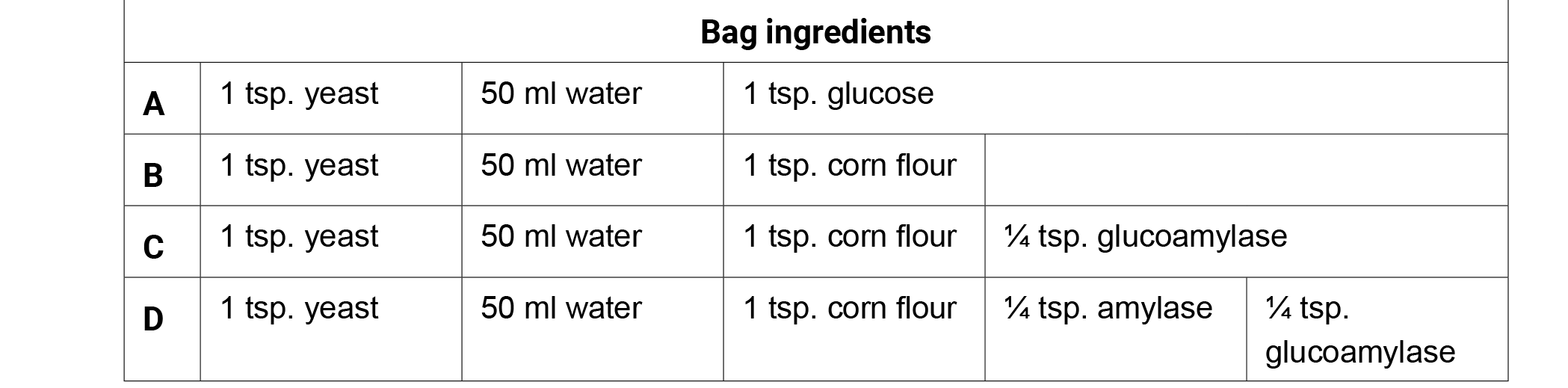
- Give each student one copy of the Fermentation Factories handout.
- Ask students, "What is occurring in each of the four bags?" Write the ingredients of each bag on the board and have students brainstorm observations or questions surrounding the function of each ingredient individually for 1 minute. Have them record both the bag contents and their observations on their charts for later use.
- Have the students share their observations in a small group for three minutes. Generate class discussion by asking groups to share their observations with the class. Possible observations or questions about the Corn Fermentation in a Bag ingredients.
- Glucose is a simple sugar (monosaccharide).
- Yeast are organisms/decomposers that eat sugars.
- Starch is a complex sugar (polysaccharide).
- Fermentation occurs when yeast consume sugar (glucose) and produce alcohol (ethanol) and carbon dioxide.
- Bag A produced the most CO2 in 20 minutes (glucose).
- Bags B and C produced very little CO2 in 20 minutes.
- Bag D produced the second largest amount of CO2 in 20 minutes.
- What do amylase and glucoamylase do? How do they function with sugars or yeast?
- Copy the problem below to give to students or read to students:
- Human consumption of fuels is on the rise as both population and affluence steadily increase. Renewable fuels, such as ethanol, can help to decrease the need for non-renewable fuel sources such as gasoline refined from crude oil. In addition, ethanol has replaced methyl tertiary-butyl ether (MTBE) as the major octane source in gasoline, which has resulted in gasohol blends of up to 10% in almost every pump in the United States. Ethanol is a renewable fuel source that is both energy positive, which means it generates more potential energy than it consumes, and helps to reduce greenhouse gas emissions. More cars are on the road than ever before, so we need to be able to produce high-quality ethanol quickly and efficiently to fuel the increase of active automobiles. Fermentation is an anaerobic process where yeast consume sugars to produce alcohol and carbon dioxide. Ethanol is created when yeast consume glucose (simple sugar). Ethanol in the United States is produced by breaking down corn flour to create glucose, which is then consumed by yeast to produce CO2, ethanol, and distillers grains. Distillers grains are the leftover corn fiber, protein, and oil that result from the breakdown of starch in corn. Here is the equation for the fermentation of glucose into ethanol and carbon dioxide.
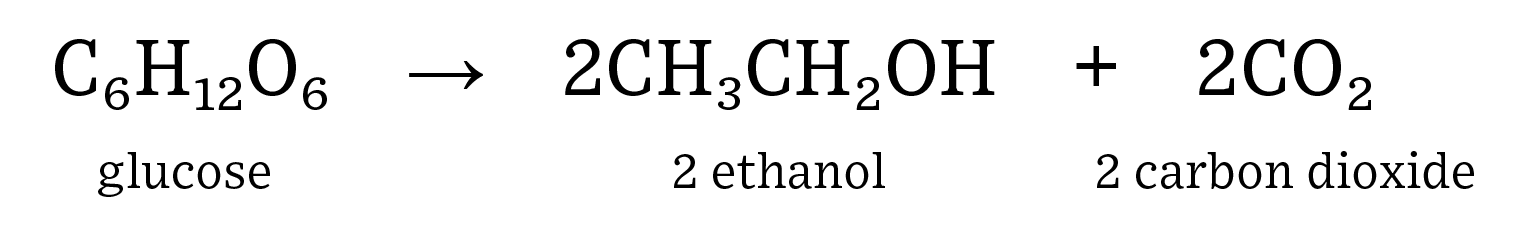
- Human consumption of fuels is on the rise as both population and affluence steadily increase. Renewable fuels, such as ethanol, can help to decrease the need for non-renewable fuel sources such as gasoline refined from crude oil. In addition, ethanol has replaced methyl tertiary-butyl ether (MTBE) as the major octane source in gasoline, which has resulted in gasohol blends of up to 10% in almost every pump in the United States. Ethanol is a renewable fuel source that is both energy positive, which means it generates more potential energy than it consumes, and helps to reduce greenhouse gas emissions. More cars are on the road than ever before, so we need to be able to produce high-quality ethanol quickly and efficiently to fuel the increase of active automobiles. Fermentation is an anaerobic process where yeast consume sugars to produce alcohol and carbon dioxide. Ethanol is created when yeast consume glucose (simple sugar). Ethanol in the United States is produced by breaking down corn flour to create glucose, which is then consumed by yeast to produce CO2, ethanol, and distillers grains. Distillers grains are the leftover corn fiber, protein, and oil that result from the breakdown of starch in corn. Here is the equation for the fermentation of glucose into ethanol and carbon dioxide.
- Read the challenge to the students: Create the greatest volume of ethanol (measured by the volume of carbon dioxide generated) in the shortest time possible. Students should work in groups of 2–3 individuals for this challenge. Review the criteria and constraints for the challenge.
- Have students observe the following criteria and constraints
- Plan an experiment/several experiments to produce ethanol in a small bag environment.
- Students may only use the following materials/amounts provided by the teacher.
- Students may have 1 or more class period(s) to experiment on the initial design(s) based on their group plan.
- Data must be collected and analyzed to provide evidence for an explanation and future design solution.
- Students report back to the class and provide future design solutions as a result of your current explanation.
- Discuss the engineering design process with your students. Encourage the student groups to create two or more experimental designs based upon their knowledge of what occurs in the phenomena bags. Why are they investigating their design? What is their reasoning for their materials? What patterns do they expect to see? They will also need to create a method for measuring their CO2 gas. We suggest that they measure volume by height displacement using a clipboard and ruler to demonstrate their volume change in CO2 gas. Students should be able to predict the outcome of some of their experimental designs based upon previous background knowledge and their observations of the anchoring phenomena.
- Encourage the students to create charts and graphs to show the volume change within their bags over time. Students should create their own experimental procedure to collect and record data.
- What are some physical ways your group can measure carbon dioxide production and/or ethanol production?
- How can your group predict the amount of carbon dioxide and ethanol that is generated from your fermentation bags?
- Students should be able to reflect on the following questions to construct their explanation below.
- What is the purpose/role of each component in your group’s fermentation bag design(s)? How did each component act upon another? Write/draw your most efficient design below. Possible answers: glucose, honey, other sugars provide food source for yeast, enzymes help to speed up fermentation, yeast are necessary to breakdown/metabolize food source into CO2 and ethanol.
- What evidence did your group generate to clarify the role of each component in your
group’s design? Possible answers: controlled variables, made comparisons between bags, left out components to see results. - What are the reactants and products of your fermentation reaction? Possible answers: reactants include sugar type and enzymes; products include CO and ethanol.
- Give students an opportunity to redesign their experiment. Assign research to determine what food sources might be best for yeast, if there are other organisms that are more efficient at fermentation and/or other enzymes that might be used. Following the research, ask students to discuss improvements of their experimental design based upon the research presented. What could they improve upon: Materials used?
Experimental conditions? Sample questions might be used to fuel the discussion- Can you create a more efficient design using different materials?
- Can you predict the outcome of other experimental designs?
- How can you change your original design to become more efficient by changing the experimental conditions?
- Make predictions using all of the available feedstocks in separate designs to determine which one will generate the most carbon dioxide and ethanol over time.
- Assign assessment: Write two to three paragraphs to construct an explanation of the fermentation process of corn into ethanol. Include what you learned about fermentation by using the evidence collected in your experiments.
Activity 2: Ticketase
What roles do different enzymes play in the fermentation of starch? How do enzymes act upon complex sugars like starch? What is the rate of enzyme activity for Ticketase and Glucoticketase? Does enzyme or substrate concentration affect the rate of enzymatic activity?
- Give each student one copy of the Ticketase student handout.
- Remind the students of the 4 fermentation bags used in Activity 1. What was occurring in each of the four bags? You can help guide the students’ discussion by asking questions as you record their observations/questions.
- How did the amylase and/or glucoamylase impact the fermentation reaction?
- What are the roles of amylase and glucoamylase?
- Did amylase/glucoamylase help the fermentation process occur more slowly, rapidly, or have no net effect?
- Have students work together in groups of 5. Provide each group with 4 strings of 50 connected tickets. Instructions for the activity are included on the student page.
- Refer to the Ticketase Teacher Key for answers to the handout, ideas for differentiation, and a rubric for assessment.
Activity 3: Biomass to Sugars
Which feedstock will produce the most glucose for fermentation?
- Prior to class, gather lab materials for students to use. Prepare amylase and glucoamylase solutions.
- Give each student one copy of the Biomass to Sugars student handout.
- Divide the class into groups of 4 students and instruct students to follow the lab instructions found on their handout. Students will set up their investigation and test for three days.
- Following the investigation, students will analyze their results to determine which conditions led to the most glucose production based on measurements using glucose test strips or glucose monitors.
- Refer to the Biomass to Sugars Teacher Key for answers to the handout, ideas for differentiation, and a rubric for assessment.
Activity 4: Macromolecules and Fuel
How do the processes of fermentation and distillation alter the macromolecule content of the feedstock and fermentation products?
- Give each student one copy of the Macromolecules and Fuel student handout.
- Divide students into groups of 2-4. Instruct them to first complete the nutrient analysis tests outlined in the "Day 1" section of the lesson. The tests should be conducted again on day 3 after fermentation, and again after the distillation process.
- Note: A Bradford Assay using Coomassie dye may be used to determine the change in protein content, a more sensitive test that can be used with the solution alone in place of the Biuret, or as an assay using a spectrophotometer to compare to a standard curve.
- Refer to the Macromolecules and Fuel Teacher Key for answers to the handout, ideas for differentiation, and a rubric for assessment.
Activity 5: Corn Mash and Distillation
How is ethanol produced? What are the steps in ethanol production?
- Prepare lab materials ahead of time for students. Make sure to create the amylase and glucoamylase solutions before the lab.
- Give each student one copy of the Corn Mash and Distillation student handout.
- Divide students into groups of 3-4 students to create a corn mash and distill the filtrate into ethanol. Carefully observe safety procedures during the lab!
- See student lesson for detailed instructions of the corn mash preparation and ethanol distillation. This lesson will take one day to prepare the corn mash, three days in between to allow for complete fermentation (this could be shorter if samples are kept warm in an incubator set to 32° C/90° F (optimal temperature for yeast metabolism). Distillation will take longer than a class period, so a demo could be prepared so that students can test the final product in the following class period.
- Safety note: Do not use any flames in the room during ethanol distillation. Students should make sure that the joints of the distillation equipment are tightly sealed in order to capture the distilled ethanol and prohibit it from being released into the atmosphere.
- Alcohol Flame Test Safety Note: This test should only be done in an active fume hood on a watch glass after the ethanol distillation is complete and there is no vapor in the air. Students will be testing the ethanol distillate for alcohol concentration by lighting it on fire. The longer the flame burns, the greater the alcohol concentration. If the distillate does not burn, the water concentration is too high (over 50%).
- Salt wash (optional): Students can separate remaining water from the ethanol distillate by adding a small amount of potassium carbonate, K2CO3, which is soluble in water but not in ethanol, to the distillate. The K2CO3 and water will form an alkaline solution and separate from the ethanol to form a dense, bottom layer with the ethanol remaining in the top layer. This can be seen more easily if a drop of food coloring is added to the distillate at the same time as the K2CO3. Students can then remove the ethanol and test it either by flame test in the fume hood, with an ethanol probe, alcohol refractometer, or in a stirling engine.
- Ask the following questions after students have complete the lab.
- How can we modify dent corn to make glucose available for fermentation?
- What role does anaerobic respiration play as yeast consume glucose to create ethanol and carbon dioxide?
- What change does glucose undergo to become ethanol and carbon dioxide?
- Refer to the Corn Mash and Distillation Teacher Key for answers to the handout, ideas for differentiation, and a rubric for assessment.
Activity 6: Biofuels from Plant Oils
How do plant oils become fuel?
- Give each student one copy of the Biofuels from Plant Oils student handout.
- This lab may be completed using either separatory funnels suspended on ring stands or glass jars. The difference will be in the way the layer of glycerol will be removed. In separatory funnels, the stop cocks can be opened to allow the glycerol and waste from washing to flow out of the bottom. If using glass jars, the waste must be removed with a serological pump with pipettes. Both sodium hydroxide and potassium hydroxide work in this procedure when mixed with methanol to make methoxide. HEET, a fuel additive available from auto part stores and most discount stores, may be substituted for methanol.
- Ask students to tell you what they know about the various types of diesel fuels (biodiesel, renewable diesel and petroleum diesel). Have them choose one of the fuels to research: where does it come from, how is it created/refined, what is it being used for, what benefit does it provide over the other forms of fuel, estimated supply, etc.
- Begin setting up for the lab by determining which materials you will use.
- Divide students into groups to create biomass-based diesel using one or more types of plant oil.
- Detailed instructions are included on the student handout. Safety notes:
- Methanol should be handled under a fume hood or in a well-ventilated area if a fume hood is not available.
- Sodium and potassium hydroxide can be caustic, so use caution when handling or provide gloves for students.
- Keep the hydroxide tightly capped; otherwise, it will attract moisture from the surrounding air.
- This activity will take 2–3 days depending on how long you allow in between washings. Students can be completing research on fuels while waiting for settling.
- Refer to the Biofuels from Plant Oils Teacher Key for answers to the handout, ideas for differentiation, and a rubric for assessment.
Elaborate
Explore graphs and charts from the National Corn Growers Association to see where corn is grown, corn yield statistics, what corn is used for, and more.
Evaluate
After conducting these activities, review and summarize the following key concepts.
- Ethanol is produced using the process of fermentation.
- Enzymes break molecules like starch into smaller molecules like disaccharides and monosaccharides that can be used for the fermentation of sugar.
- Macromolecules are present in different amounts before, during, and after fermentation of corn flour into ethanol.
Sources
Acknowledgements
This lesson is an excerpt of the Energy and Biofuels unit created by Nourish the Future.